Solving the Challenges of a Complex Cardiac Diagnostic Device
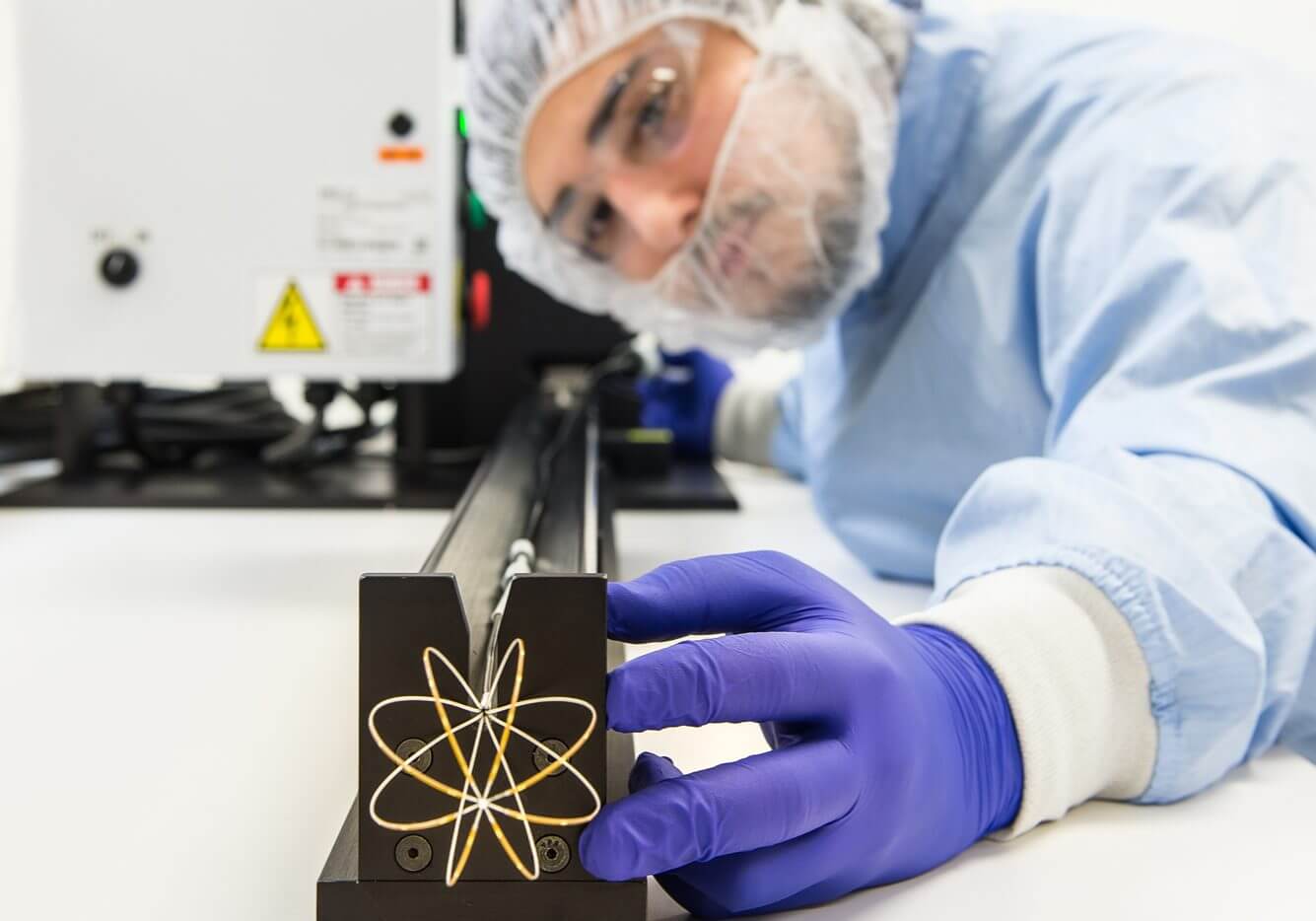
Case Study: Manufacturing an Intricate Atrial Fibrillation Diagnostic Device
Overview
SMC partnered with an OEM to manufacture a highly complex atrial fibrillation vascular catheter capable of detecting 64 AFib signals simultaneously through a global array. The device integrates these signals to deliver cutting-edge cardiac diagnostics, requiring extreme precision, micro-assembly expertise, and tightly controlled material processes.
The Challenge
The Mapping Catheter program presented significant technical and manufacturing hurdles due to the device’s micro-scale circuitry and stringent performance requirements. Key challenges included:
- Microscopic, high-density circuitry requiring exceptional precision
- Redesigned device architecture focused on sustainable manufacturability
- Delicate, highly flexible components requiring skilled manual assembly
- Materials that demanded narrow, tightly controlled process windows
- Custom-built programming tools for circuit analysis and verification
- Documentation-heavy qualification and verification processes essential for device reliability
The Solution
SMC developed and executed a comprehensive manufacturing strategy combining skilled craftsmanship, advanced technology, and scientific material insights. The solution included:
- A broad-based operator training and assessment program to support highly specialized manual assembly
- Progressive surface texture modifications to improve performance
- Advanced welding technology delivering high reliability
- Micro-soldering processes optimized for copper bonding
- Customized laser housing for enhanced precision
- Use of research to refine alloy balance for improved functionality
- Advanced adhesive application systems to improve quality and repeatability
- Improved reflow control for consistent, reliable connections
- A custom-built circuit analyzer to evaluate circuit integrity and ensure robust electrical performance
The Results
SMC’s engineering, materials, and micro-manufacturing expertise enabled the successful commercialization of a highly advanced cardiac mapping catheter. The collaboration delivered:
- A fully manufacturable, highly intricate device capable of capturing 64 AFib signals
- Improved assembly reliability and repeatability through advanced welding, soldering, and adhesive systems
- Enhanced material performance and circuit integrity through controlled processes and custom testing tools
- A trained, validated assembly workforce equipped to build a device of exceptional complexity